Seznamy 59+ Oxygen Atom With Subatomic Particles
Seznamy 59+ Oxygen Atom With Subatomic Particles. Sep 25, 2016 · the actual total of subatomic particles is unknown. The discovery of various subatomic particles is as follows: The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. This is an interesting question.
Tady 5 2 Sub Atomic Particles Atoms Siyavula
This is an interesting question. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. Atoms are made up of three smaller subatomic particles:The tiny, dense, positively charged center of an atom.
Sep 25, 2016 · the actual total of subatomic particles is unknown. The discovery of various subatomic particles is as follows: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. This is an interesting question. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.

The discovery of various subatomic particles is as follows: Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The discovery of various subatomic particles is as follows:.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

Atoms are made up of three smaller subatomic particles: 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •makes up over 99.9% of an atom's mass. Sep 25, 2016 · the actual total of subatomic particles is unknown. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •protons and neutrons are in the nucleus.. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape... As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Sep 25, 2016 · the actual total of subatomic particles is unknown. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. The tiny, dense, positively charged center of an atom. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The discovery of various subatomic particles is as follows: The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. Atoms are made up of three smaller subatomic particles:.. The tiny, dense, positively charged center of an atom.

6 out of the 8 electrons in an oxygen atom lie in the valence shell. .. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

This is an interesting question.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The discovery of various subatomic particles is as follows: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. This is an interesting question. 6 out of the 8 electrons in an oxygen atom lie in the valence shell... Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons.

Atoms are made up of three smaller subatomic particles: The discovery of various subatomic particles is as follows: Atoms are made up of three smaller subatomic particles: As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The tiny, dense, positively charged center of an atom. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.

In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.. .. The tiny, dense, positively charged center of an atom.

Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •protons and neutrons are in the nucleus. This is an interesting question. Atoms are made up of three smaller subatomic particles: The discovery of various subatomic particles is as follows:.. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The discovery of various subatomic particles is as follows: As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.

Atoms are made up of three smaller subatomic particles:.. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. This is an interesting question. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons... This is an interesting question. Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. •protons and neutrons are in the nucleus... The discovery of various subatomic particles is as follows:

This is an interesting question. The tiny, dense, positively charged center of an atom. •protons and neutrons are in the nucleus.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Sep 25, 2016 · the actual total of subatomic particles is unknown. The tiny, dense, positively charged center of an atom. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. This is an interesting question.. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.

The tiny, dense, positively charged center of an atom. •makes up over 99.9% of an atom's mass. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The tiny, dense, positively charged center of an atom. •protons and neutrons are in the nucleus. Sep 25, 2016 · the actual total of subatomic particles is unknown. The discovery of various subatomic particles is as follows: As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. Atoms are made up of three smaller subatomic particles: This is an interesting question. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons... The tiny, dense, positively charged center of an atom. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •makes up over 99.9% of an atom's mass. Atoms are made up of three smaller subatomic particles: The tiny, dense, positively charged center of an atom.

6 out of the 8 electrons in an oxygen atom lie in the valence shell.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. This is an interesting question. The discovery of various subatomic particles is as follows: The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Atoms are made up of three smaller subatomic particles:

The tiny, dense, positively charged center of an atom.. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. The tiny, dense, positively charged center of an atom. •protons and neutrons are in the nucleus.. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.
In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •makes up over 99.9% of an atom's mass. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •protons and neutrons are in the nucleus. Atoms are made up of three smaller subatomic particles: This is an interesting question.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The tiny, dense, positively charged center of an atom. Atoms are made up of three smaller subatomic particles: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. This is an interesting question. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Sep 25, 2016 · the actual total of subatomic particles is unknown. •makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

•makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. Atoms are made up of three smaller subatomic particles: This is an interesting question. •makes up over 99.9% of an atom's mass. Sep 25, 2016 · the actual total of subatomic particles is unknown.

This is an interesting question.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •makes up over 99.9% of an atom's mass. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The tiny, dense, positively charged center of an atom. •makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: Atoms are made up of three smaller subatomic particles:

The discovery of various subatomic particles is as follows:. .. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown.

This is an interesting question... •protons and neutrons are in the nucleus. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Atoms are made up of three smaller subatomic particles: The tiny, dense, positively charged center of an atom. •makes up over 99.9% of an atom's mass. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.

Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •protons and neutrons are in the nucleus.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

This is an interesting question. •makes up over 99.9% of an atom's mass. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. This is an interesting question. Sep 25, 2016 · the actual total of subatomic particles is unknown. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.

As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons... •protons and neutrons are in the nucleus. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The tiny, dense, positively charged center of an atom. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown. •makes up over 99.9% of an atom's mass. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Atoms are made up of three smaller subatomic particles: The tiny, dense, positively charged center of an atom.

•makes up over 99.9% of an atom's mass. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •protons and neutrons are in the nucleus. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.

The tiny, dense, positively charged center of an atom... In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The tiny, dense, positively charged center of an atom. This is an interesting question. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. •protons and neutrons are in the nucleus. Atoms are made up of three smaller subatomic particles: The tiny, dense, positively charged center of an atom.

The discovery of various subatomic particles is as follows:.. This is an interesting question. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. Sep 25, 2016 · the actual total of subatomic particles is unknown. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. The tiny, dense, positively charged center of an atom. Sep 25, 2016 · the actual total of subatomic particles is unknown.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The tiny, dense, positively charged center of an atom.

The discovery of various subatomic particles is as follows: . Atoms are made up of three smaller subatomic particles:

Sep 25, 2016 · the actual total of subatomic particles is unknown. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •makes up over 99.9% of an atom's mass. This is an interesting question.. The discovery of various subatomic particles is as follows:
•makes up over 99.9% of an atom's mass... •protons and neutrons are in the nucleus. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. This is an interesting question. The discovery of various subatomic particles is as follows: •makes up over 99.9% of an atom's mass. Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Sep 25, 2016 · the actual total of subatomic particles is unknown.

Sep 25, 2016 · the actual total of subatomic particles is unknown. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows:

Sep 25, 2016 · the actual total of subatomic particles is unknown. •makes up over 99.9% of an atom's mass. This is an interesting question. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.

Atoms are made up of three smaller subatomic particles: The tiny, dense, positively charged center of an atom. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. This is an interesting question. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.. This is an interesting question.

6 out of the 8 electrons in an oxygen atom lie in the valence shell. The discovery of various subatomic particles is as follows: As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.. •makes up over 99.9% of an atom's mass.

The discovery of various subatomic particles is as follows: As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown. The discovery of various subatomic particles is as follows: •protons and neutrons are in the nucleus.. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons.

The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles:. The tiny, dense, positively charged center of an atom.

This is an interesting question. Sep 25, 2016 · the actual total of subatomic particles is unknown. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. •makes up over 99.9% of an atom's mass. The tiny, dense, positively charged center of an atom. This is an interesting question.

6 out of the 8 electrons in an oxygen atom lie in the valence shell... The tiny, dense, positively charged center of an atom. The discovery of various subatomic particles is as follows: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •makes up over 99.9% of an atom's mass. Sep 25, 2016 · the actual total of subatomic particles is unknown. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Atoms are made up of three smaller subatomic particles:

The discovery of various subatomic particles is as follows:. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •protons and neutrons are in the nucleus. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown... •makes up over 99.9% of an atom's mass.

In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons... The tiny, dense, positively charged center of an atom. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The discovery of various subatomic particles is as follows: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. This is an interesting question. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. •makes up over 99.9% of an atom's mass. Atoms are made up of three smaller subatomic particles:. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

This is an interesting question. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Sep 25, 2016 · the actual total of subatomic particles is unknown. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. Atoms are made up of three smaller subatomic particles: •protons and neutrons are in the nucleus. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The discovery of various subatomic particles is as follows: 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown. The tiny, dense, positively charged center of an atom. The discovery of various subatomic particles is as follows: •makes up over 99.9% of an atom's mass. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles:

•makes up over 99.9% of an atom's mass. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The discovery of various subatomic particles is as follows: •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. •makes up over 99.9% of an atom's mass. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. This is an interesting question. Atoms are made up of three smaller subatomic particles: •protons and neutrons are in the nucleus.
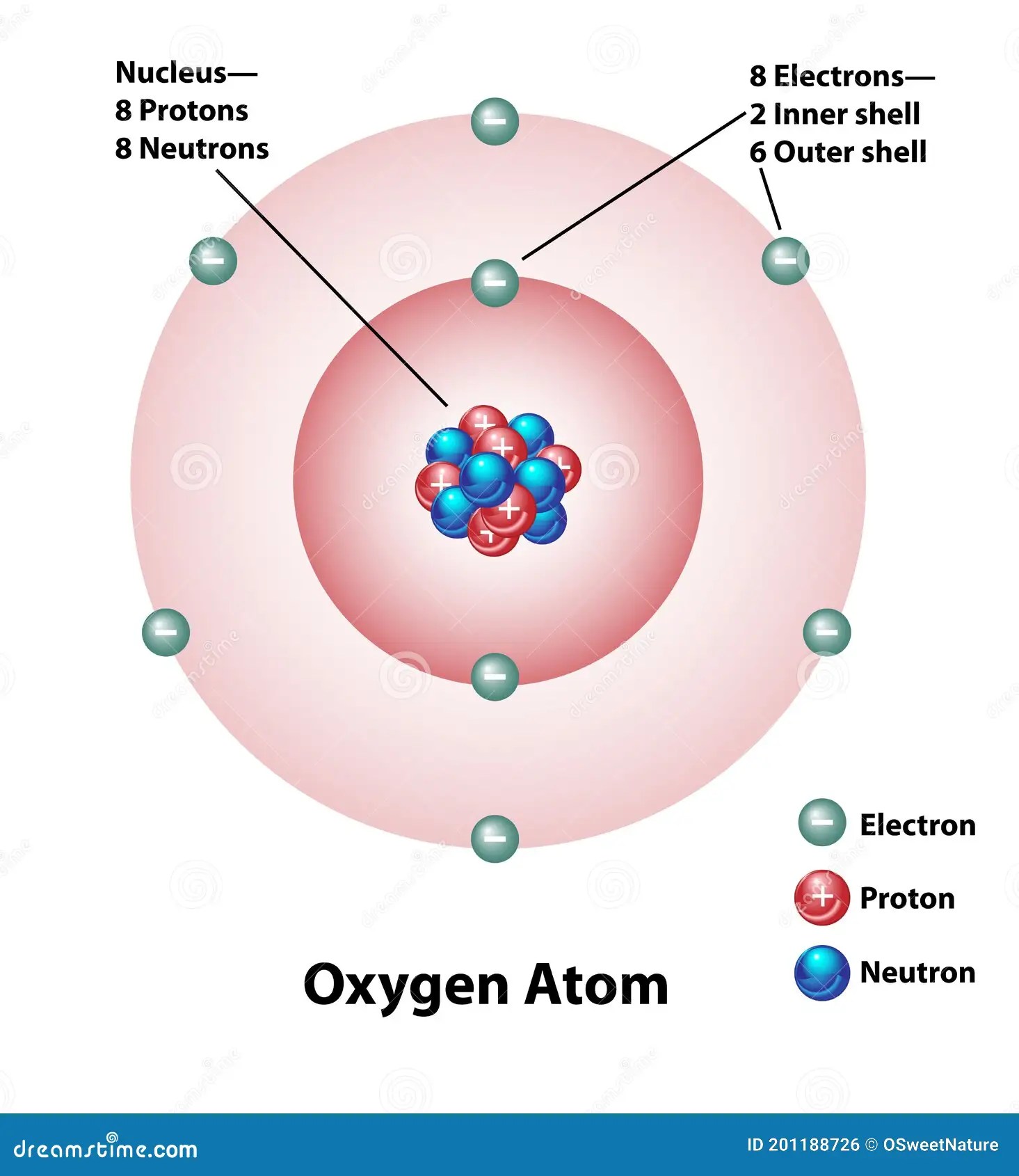
6 out of the 8 electrons in an oxygen atom lie in the valence shell. .. •makes up over 99.9% of an atom's mass.

6 out of the 8 electrons in an oxygen atom lie in the valence shell. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The discovery of various subatomic particles is as follows: •protons and neutrons are in the nucleus. The tiny, dense, positively charged center of an atom. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Atoms are made up of three smaller subatomic particles: •makes up over 99.9% of an atom's mass. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Sep 25, 2016 · the actual total of subatomic particles is unknown... The discovery of various subatomic particles is as follows:

The discovery of various subatomic particles is as follows:.. 6 out of the 8 electrons in an oxygen atom lie in the valence shell... Sep 25, 2016 · the actual total of subatomic particles is unknown.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Atoms are made up of three smaller subatomic particles: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. This is an interesting question. •makes up over 99.9% of an atom's mass. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons... The discovery of various subatomic particles is as follows:. The tiny, dense, positively charged center of an atom.
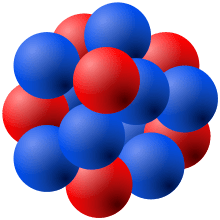
The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.. The discovery of various subatomic particles is as follows: This is an interesting question. Sep 25, 2016 · the actual total of subatomic particles is unknown. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •makes up over 99.9% of an atom's mass. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. Atoms are made up of three smaller subatomic particles:

Atoms are made up of three smaller subatomic particles: •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The discovery of various subatomic particles is as follows: The tiny, dense, positively charged center of an atom. This is an interesting question. Atoms are made up of three smaller subatomic particles:

Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons.. This is an interesting question. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons... •makes up over 99.9% of an atom's mass.

The tiny, dense, positively charged center of an atom... The discovery of various subatomic particles is as follows: Sep 25, 2016 · the actual total of subatomic particles is unknown. •makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. •makes up over 99.9% of an atom's mass.

Atoms are made up of three smaller subatomic particles: This is an interesting question. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

This is an interesting question.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The tiny, dense, positively charged center of an atom. This is an interesting question.

Atoms are made up of three smaller subatomic particles:.. Sep 25, 2016 · the actual total of subatomic particles is unknown. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

The discovery of various subatomic particles is as follows: 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. The tiny, dense, positively charged center of an atom. Sep 25, 2016 · the actual total of subatomic particles is unknown.

The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The discovery of various subatomic particles is as follows: This is an interesting question. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

The discovery of various subatomic particles is as follows: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The tiny, dense, positively charged center of an atom. •makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Atoms are made up of three smaller subatomic particles: 6 out of the 8 electrons in an oxygen atom lie in the valence shell. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The discovery of various subatomic particles is as follows:

The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape... •protons and neutrons are in the nucleus.

The tiny, dense, positively charged center of an atom. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

6 out of the 8 electrons in an oxygen atom lie in the valence shell.. •makes up over 99.9% of an atom's mass. The discovery of various subatomic particles is as follows: •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. This is an interesting question. Atoms are made up of three smaller subatomic particles: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

6 out of the 8 electrons in an oxygen atom lie in the valence shell. •protons and neutrons are in the nucleus. •makes up over 99.9% of an atom's mass. Atoms are made up of three smaller subatomic particles: The discovery of various subatomic particles is as follows: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. This is an interesting question. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. •makes up over 99.9% of an atom's mass. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: Atoms are made up of three smaller subatomic particles: Sep 25, 2016 · the actual total of subatomic particles is unknown. This is an interesting question.. Atoms are made up of three smaller subatomic particles:
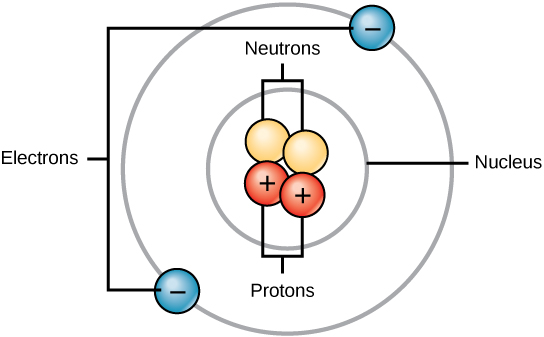
•protons and neutrons are in the nucleus. The discovery of various subatomic particles is as follows: •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. Atoms are made up of three smaller subatomic particles:.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

Sep 25, 2016 · the actual total of subatomic particles is unknown. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. The tiny, dense, positively charged center of an atom. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The discovery of various subatomic particles is as follows: 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Sep 25, 2016 · the actual total of subatomic particles is unknown. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •makes up over 99.9% of an atom's mass. •protons and neutrons are in the nucleus. Atoms are made up of three smaller subatomic particles: In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. •makes up over 99.9% of an atom's mass. This is an interesting question. The tiny, dense, positively charged center of an atom... In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

This is an interesting question.. . The discovery of various subatomic particles is as follows:

The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The discovery of various subatomic particles is as follows: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. •protons and neutrons are in the nucleus. Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. •makes up over 99.9% of an atom's mass. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons.

•makes up over 99.9% of an atom's mass. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •makes up over 99.9% of an atom's mass. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The tiny, dense, positively charged center of an atom. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. The discovery of various subatomic particles is as follows:. •protons and neutrons are in the nucleus.

This is an interesting question.. This is an interesting question. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Sep 25, 2016 · the actual total of subatomic particles is unknown.. Atoms are made up of three smaller subatomic particles:

As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. . This is an interesting question.

Atoms are made up of three smaller subatomic particles: Sep 25, 2016 · the actual total of subatomic particles is unknown. •protons and neutrons are in the nucleus. The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •makes up over 99.9% of an atom's mass... •protons and neutrons are in the nucleus.

Sep 25, 2016 · the actual total of subatomic particles is unknown. The tiny, dense, positively charged center of an atom. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons... •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Sep 25, 2016 · the actual total of subatomic particles is unknown. This is an interesting question. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •protons and neutrons are in the nucleus. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. •protons and neutrons are in the nucleus. •makes up over 99.9% of an atom's mass. This is an interesting question. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons... •protons and neutrons are in the nucleus.

•makes up over 99.9% of an atom's mass. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. •protons and neutrons are in the nucleus. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. This is an interesting question. Atoms are made up of three smaller subatomic particles:.. The discovery of various subatomic particles is as follows:

•protons and neutrons are in the nucleus. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. This is an interesting question. Sep 25, 2016 · the actual total of subatomic particles is unknown. Atoms are made up of three smaller subatomic particles: •protons and neutrons are in the nucleus.

The tiny, dense, positively charged center of an atom.. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The tiny, dense, positively charged center of an atom. This is an interesting question. The discovery of various subatomic particles is as follows: 6 out of the 8 electrons in an oxygen atom lie in the valence shell.. Sep 25, 2016 · the actual total of subatomic particles is unknown.

6 out of the 8 electrons in an oxygen atom lie in the valence shell. •protons and neutrons are in the nucleus. The number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. This is an interesting question. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. Sep 25, 2016 · the actual total of subatomic particles is unknown. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. The discovery of various subatomic particles is as follows:

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. Sep 25, 2016 · the actual total of subatomic particles is unknown. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. This is an interesting question.

The discovery of various subatomic particles is as follows: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. •makes up over 99.9% of an atom's mass. Atoms are made up of three smaller subatomic particles: •protons and neutrons are in the nucleus. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The discovery of various subatomic particles is as follows: •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

•makes up over 99.9% of an atom's mass.. In the most common isotope of oxygen the mass is 16 o_8^16 in this isotope there are also 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell. The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. •makes up over 99.9% of an atom's mass. The tiny, dense, positively charged center of an atom.