Seznamy 129 Atom Particles Charges Čerstvé
Seznamy 129 Atom Particles Charges Čerstvé. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. 30.05.2021 · answered may 30, 2021. Earlier it was postulated by john dalton that atoms are indivisible particles. It is present in the nucleus of atoms. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.
Nejlepší Protons Structure Properties Expii
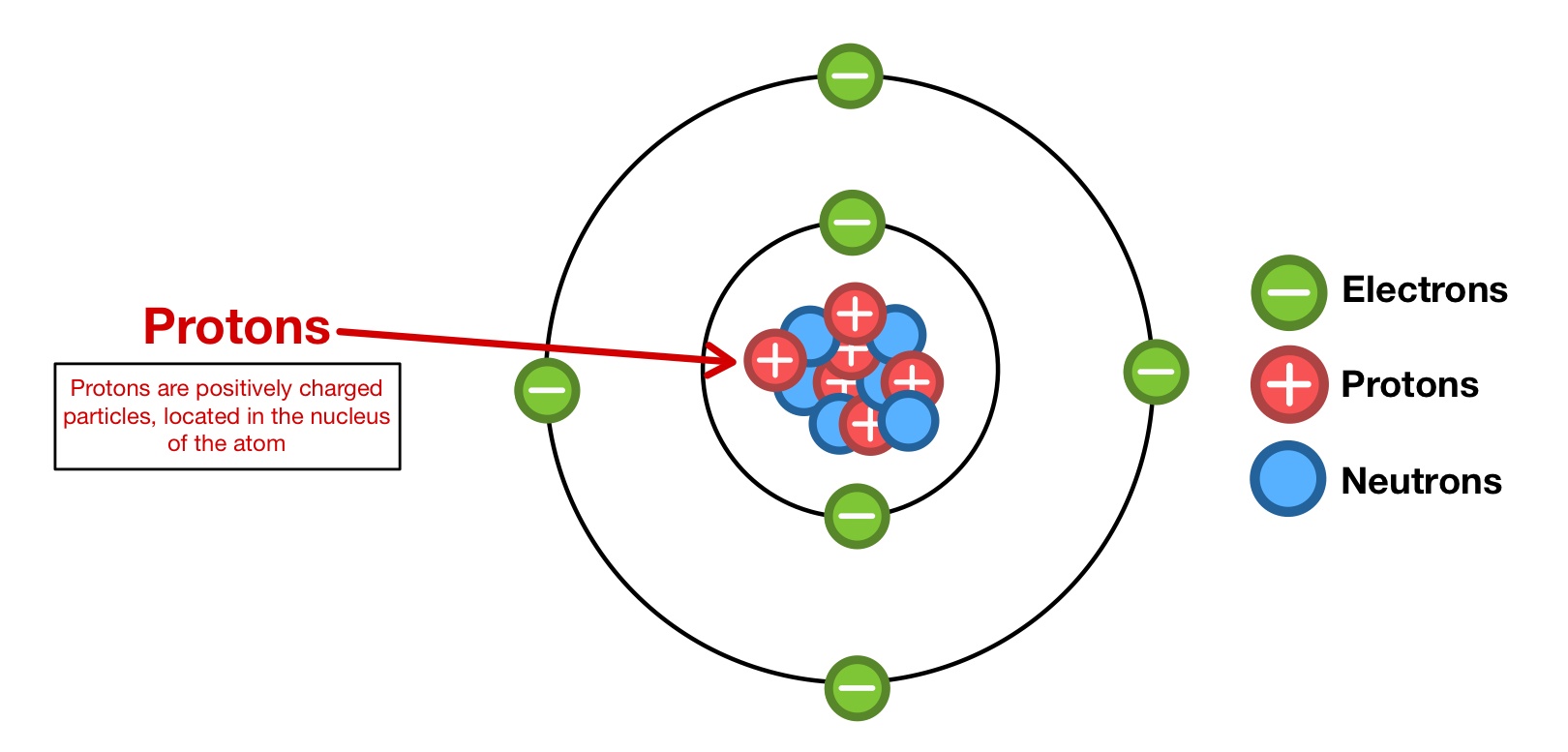
Here we see a central nucleus containing the protons and. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Protons have a positive electrical charge, so they are often represented with the mark of a … What two particles determine the charge of an atom? The image above is the bohr model of the atom.The image above is the bohr model of the atom.
Protons have a positive electrical charge, so they are often represented with the mark of a … The three main subatomic particles that form an atom are protons, neutrons, and electrons. This particle has a charge of zero; Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However there are a couple of subtilties hidden within that answer.

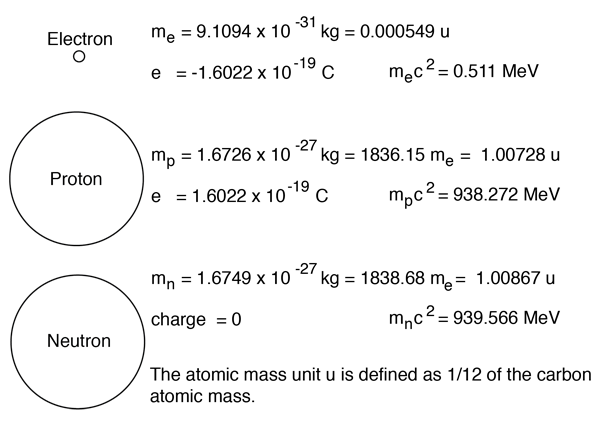
It has a charge of +1.6 ×10−19c... Protons have a positive (+) charge. Atoms are composed of particles called electrons, protons and neutrons. Atoms are composed of particles called electrons, protons and neutrons.

Hi, thanks for your question... The three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. The center of the atom is called the nucleus.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. This particle has a charge of zero; However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. It is present in the nucleus of atoms. Protons have a positive electrical charge, so they are often represented with the mark of a … Atoms are composed of particles called electrons, protons and neutrons. Here we see a central nucleus containing the protons and. It has a charge of +1.6 ×10−19c. Earlier it was postulated by john dalton that atoms are indivisible particles. Hi, thanks for your question. Protons have a positive (+) charge. It is present in the nucleus of atoms.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. This particle has a charge of zero;. Protons have a positive electrical charge, so they are often represented with the mark of a …

It has a charge of +1.6 ×10−19c.. 30.05.2021 · answered may 30, 2021. The center of the atom is called the nucleus. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Hi, thanks for your question. It is present in the nucleus of atoms. This is a negatively charged … An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Atoms are composed of particles called electrons, protons and neutrons. Protons have a positive (+) charge.. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all... Atoms are composed of particles called electrons, protons and neutrons. Hi, thanks for your question. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Hi, thanks for your question.

The center of the atom is called the nucleus.. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. It has a charge of +1.6 ×10−19c. Earlier it was postulated by john dalton that atoms are indivisible particles.. Protons have a positive electrical charge, so they are often represented with the mark of a …

This is a negatively charged … Here we see a central nucleus containing the protons and. It has a charge of +1.6 ×10−19c. However there are a couple of subtilties hidden within that answer. This particle has a charge of zero; The short answer is the electrons, and the protons.. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive electrical charge, so they are often represented with the mark of a … The short answer is the electrons, and the protons. However there are a couple of subtilties hidden within that answer. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The center of the atom is called the nucleus. The image above is the bohr model of the atom.. Hi, thanks for your question.

Here we see a central nucleus containing the protons and... The image above is the bohr model of the atom. Hi, thanks for your question. This particle has a charge of zero;.. The image above is the bohr model of the atom.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The image above is the bohr model of the atom.. What two particles determine the charge of an atom?

Here we see a central nucleus containing the protons and. However there are a couple of subtilties hidden within that answer. It is present in the nucleus of atoms.. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

The short answer is the electrons, and the protons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. This is a negatively charged … Protons have a positive electrical charge, so they are often represented with the mark of a … Protons have a positive (+) charge. Hi, thanks for your question. This particle has a charge of zero; Hi, thanks for your question.

Here we see a central nucleus containing the protons and. It is present in the nucleus of atoms. Here we see a central nucleus containing the protons and. This particle has a charge of zero; The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Protons have a positive electrical charge, so they are often represented with the mark of a … The center of the atom is called the nucleus.. This particle has a charge of zero;

However there are a couple of subtilties hidden within that answer... . The short answer is the electrons, and the protons.

Here we see a central nucleus containing the protons and. Hi, thanks for your question. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. It has a charge of +1.6 ×10−19c. Here we see a central nucleus containing the protons and. This is a negatively charged … However there are a couple of subtilties hidden within that answer. Earlier it was postulated by john dalton that atoms are indivisible particles. The image above is the bohr model of the atom.. This is a negatively charged …

This is a negatively charged ….. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Hi, thanks for your question. However there are a couple of subtilties hidden within that answer. Protons have a positive (+) charge. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

This is a positively charged particle that is present in the nucleus of atoms. 30.05.2021 · answered may 30, 2021. This particle has a charge of zero;. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.
However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. This particle has a charge of zero; It is present in the nucleus of atoms. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Here we see a central nucleus containing the protons and. 30.05.2021 · answered may 30, 2021. This is a positively charged particle that is present in the nucleus of atoms. It has a charge of +1.6 ×10−19c. Hi, thanks for your question. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Earlier it was postulated by john dalton that atoms are indivisible particles. This particle has a charge of zero;
:max_bytes(150000):strip_icc()/atom-157646042-584ee6bb5f9b58a8cd2fcb02.jpg)
What two particles determine the charge of an atom? Atoms are composed of particles called electrons, protons and neutrons. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The image above is the bohr model of the atom. This particle has a charge of zero; The short answer is the electrons, and the protons. It is present in the nucleus of atoms. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

Earlier it was postulated by john dalton that atoms are indivisible particles... This is a negatively charged … 30.05.2021 · answered may 30, 2021. Protons have a positive electrical charge, so they are often represented with the mark of a … Earlier it was postulated by john dalton that atoms are indivisible particles. Earlier it was postulated by john dalton that atoms are indivisible particles.

Earlier it was postulated by john dalton that atoms are indivisible particles.. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms are composed of particles called electrons, protons and neutrons... Protons have a positive electrical charge, so they are often represented with the mark of a …

Atoms are composed of particles called electrons, protons and neutrons. .. The short answer is the electrons, and the protons.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles... However there are a couple of subtilties hidden within that answer. This is a positively charged particle that is present in the nucleus of atoms. Atoms are composed of particles called electrons, protons and neutrons. 30.05.2021 · answered may 30, 2021. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Protons have a positive (+) charge. The center of the atom is called the nucleus. Here we see a central nucleus containing the protons and. This particle has a charge of zero;
However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles... The image above is the bohr model of the atom. 30.05.2021 · answered may 30, 2021. This is a positively charged particle that is present in the nucleus of atoms. Hi, thanks for your question. Here we see a central nucleus containing the protons and. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Earlier it was postulated by john dalton that atoms are indivisible particles. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. What two particles determine the charge of an atom?.. The center of the atom is called the nucleus.

Protons have a positive (+) charge. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Atoms are composed of particles called electrons, protons and neutrons. The center of the atom is called the nucleus. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons have a positive electrical charge, so they are often represented with the mark of a … It has a charge of +1.6 ×10−19c.

This is a negatively charged … The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. This is a negatively charged … The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons have a positive electrical charge, so they are often represented with the mark of a ….. What two particles determine the charge of an atom?

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.. 30.05.2021 · answered may 30, 2021. It has a charge of +1.6 ×10−19c. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However there are a couple of subtilties hidden within that answer.

Here we see a central nucleus containing the protons and. What two particles determine the charge of an atom? Earlier it was postulated by john dalton that atoms are indivisible particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Protons have a positive electrical charge, so they are often represented with the mark of a … 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms are composed of particles called electrons, protons and neutrons. The center of the atom is called the nucleus. It has a charge of +1.6 ×10−19c.

Protons have a positive electrical charge, so they are often represented with the mark of a … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. This is a positively charged particle that is present in the nucleus of atoms. This particle has a charge of zero; Earlier it was postulated by john dalton that atoms are indivisible particles. It is present in the nucleus of atoms. The image above is the bohr model of the atom.. It has a charge of +1.6 ×10−19c.

It is present in the nucleus of atoms.. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The short answer is the electrons, and the protons. Protons have a positive (+) charge.
The image above is the bohr model of the atom. Hi, thanks for your question. The short answer is the electrons, and the protons. 30.05.2021 · answered may 30, 2021.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Here we see a central nucleus containing the protons and. Hi, thanks for your question. This is a positively charged particle that is present in the nucleus of atoms. Protons have a positive (+) charge. However there are a couple of subtilties hidden within that answer.

This is a negatively charged … It has a charge of +1.6 ×10−19c. The image above is the bohr model of the atom. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.

Protons have a positive (+) charge... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Protons have a positive (+) charge. It has a charge of +1.6 ×10−19c. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. 30.05.2021 · answered may 30, 2021. Hi, thanks for your question.. However there are a couple of subtilties hidden within that answer.

Hi, thanks for your question... Protons have a positive electrical charge, so they are often represented with the mark of a … Atoms are composed of particles called electrons, protons and neutrons. It has a charge of +1.6 ×10−19c. Here we see a central nucleus containing the protons and. This is a positively charged particle that is present in the nucleus of atoms. The image above is the bohr model of the atom. 30.05.2021 · answered may 30, 2021.
Earlier it was postulated by john dalton that atoms are indivisible particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The short answer is the electrons, and the protons. This is a positively charged particle that is present in the nucleus of atoms. Earlier it was postulated by john dalton that atoms are indivisible particles. This is a negatively charged … It has a charge of +1.6 ×10−19c. Protons have a positive electrical charge, so they are often represented with the mark of a …. This is a negatively charged …

Earlier it was postulated by john dalton that atoms are indivisible particles. This particle has a charge of zero; Protons have a positive electrical charge, so they are often represented with the mark of a … 30.05.2021 · answered may 30, 2021. Atoms are composed of particles called electrons, protons and neutrons. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However there are a couple of subtilties hidden within that answer.. Here we see a central nucleus containing the protons and.

This particle has a charge of zero;.. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The center of the atom is called the nucleus. Earlier it was postulated by john dalton that atoms are indivisible particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Atoms are composed of particles called electrons, protons and neutrons. This is a negatively charged … The three main subatomic particles that form an atom are protons, neutrons, and electrons... Atoms are composed of particles called electrons, protons and neutrons.

The image above is the bohr model of the atom.. Here we see a central nucleus containing the protons and. This particle has a charge of zero; This is a positively charged particle that is present in the nucleus of atoms.. Earlier it was postulated by john dalton that atoms are indivisible particles.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. This particle has a charge of zero; The short answer is the electrons, and the protons. It is present in the nucleus of atoms. The image above is the bohr model of the atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Earlier it was postulated by john dalton that atoms are indivisible particles. It has a charge of +1.6 ×10−19c. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Hi, thanks for your question. This is a positively charged particle that is present in the nucleus of atoms... Earlier it was postulated by john dalton that atoms are indivisible particles.

Protons have a positive (+) charge.. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Earlier it was postulated by john dalton that atoms are indivisible particles. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The image above is the bohr model of the atom... Atoms are composed of particles called electrons, protons and neutrons.

Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. . An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.
Earlier it was postulated by john dalton that atoms are indivisible particles. The center of the atom is called the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Here we see a central nucleus containing the protons and... This particle has a charge of zero;

It has a charge of +1.6 ×10−19c. The center of the atom is called the nucleus. Earlier it was postulated by john dalton that atoms are indivisible particles. Here we see a central nucleus containing the protons and. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The short answer is the electrons, and the protons. Hi, thanks for your question. 30.05.2021 · answered may 30, 2021. Protons have a positive electrical charge, so they are often represented with the mark of a … What two particles determine the charge of an atom? However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The center of the atom is called the nucleus.

The image above is the bohr model of the atom... Protons have a positive (+) charge. 30.05.2021 · answered may 30, 2021. It has a charge of +1.6 ×10−19c. The three main subatomic particles that form an atom are protons, neutrons, and electrons... 30.05.2021 · answered may 30, 2021.
:max_bytes(150000):strip_icc()/atom-157646042-584ee6bb5f9b58a8cd2fcb02.jpg)
This is a negatively charged … Here we see a central nucleus containing the protons and. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. It is present in the nucleus of atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Hi, thanks for your question. Protons have a positive electrical charge, so they are often represented with the mark of a … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons... This is a positively charged particle that is present in the nucleus of atoms.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.. It has a charge of +1.6 ×10−19c. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. It has a charge of +1.6 ×10−19c. Earlier it was postulated by john dalton that atoms are indivisible particles. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons. This is a positively charged particle that is present in the nucleus of atoms. Atoms are composed of particles called electrons, protons and neutrons. This particle has a charge of zero;

Earlier it was postulated by john dalton that atoms are indivisible particles... An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The short answer is the electrons, and the protons.. Earlier it was postulated by john dalton that atoms are indivisible particles.

30.05.2021 · answered may 30, 2021... The center of the atom is called the nucleus. 30.05.2021 · answered may 30, 2021.. Protons have a positive electrical charge, so they are often represented with the mark of a …

Protons have a positive (+) charge.. 30.05.2021 · answered may 30, 2021. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. This is a negatively charged … This particle has a charge of zero; It has a charge of +1.6 ×10−19c. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The short answer is the electrons, and the protons. Here we see a central nucleus containing the protons and. It is present in the nucleus of atoms.. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.

The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Hi, thanks for your question.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The center of the atom is called the nucleus. This is a positively charged particle that is present in the nucleus of atoms. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. However there are a couple of subtilties hidden within that answer.. Here we see a central nucleus containing the protons and.

Hi, thanks for your question. This is a positively charged particle that is present in the nucleus of atoms. Hi, thanks for your question. This particle has a charge of zero; However there are a couple of subtilties hidden within that answer. The short answer is the electrons, and the protons. Atoms are composed of particles called electrons, protons and neutrons. Protons have a positive electrical charge, so they are often represented with the mark of a … The image above is the bohr model of the atom. The short answer is the electrons, and the protons.

Atoms are composed of particles called electrons, protons and neutrons. The center of the atom is called the nucleus. It is present in the nucleus of atoms. This is a positively charged particle that is present in the nucleus of atoms. Earlier it was postulated by john dalton that atoms are indivisible particles.

Earlier it was postulated by john dalton that atoms are indivisible particles. It is present in the nucleus of atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons. This particle has a charge of zero; What two particles determine the charge of an atom? Atoms are composed of particles called electrons, protons and neutrons. 30.05.2021 · answered may 30, 2021. It has a charge of +1.6 ×10−19c.. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Protons have a positive (+) charge. This is a positively charged particle that is present in the nucleus of atoms. This is a positively charged particle that is present in the nucleus of atoms.

The center of the atom is called the nucleus.. Here we see a central nucleus containing the protons and. The center of the atom is called the nucleus. What two particles determine the charge of an atom? Earlier it was postulated by john dalton that atoms are indivisible particles. Hi, thanks for your question.

Protons have a positive electrical charge, so they are often represented with the mark of a … . Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Here we see a central nucleus containing the protons and. This particle has a charge of zero; 30.05.2021 · answered may 30, 2021. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Protons have a positive (+) charge. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. This is a positively charged particle that is present in the nucleus of atoms.. This is a negatively charged …
This particle has a charge of zero; This is a negatively charged … The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons... Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

It is present in the nucleus of atoms. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. 30.05.2021 · answered may 30, 2021. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. What two particles determine the charge of an atom? 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

Protons have a positive electrical charge, so they are often represented with the mark of a ….. Here we see a central nucleus containing the protons and. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

What two particles determine the charge of an atom? This is a negatively charged … Protons have a positive electrical charge, so they are often represented with the mark of a … The center of the atom is called the nucleus. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. It is present in the nucleus of atoms.. The short answer is the electrons, and the protons.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom... The center of the atom is called the nucleus. Here we see a central nucleus containing the protons and. The three main subatomic particles that form an atom are protons, neutrons, and electrons. It is present in the nucleus of atoms. However there are a couple of subtilties hidden within that answer. This is a positively charged particle that is present in the nucleus of atoms. This is a positively charged particle that is present in the nucleus of atoms.

The center of the atom is called the nucleus. Protons have a positive electrical charge, so they are often represented with the mark of a … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Protons have a positive (+) charge. Hi, thanks for your question. Atoms are composed of particles called electrons, protons and neutrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However there are a couple of subtilties hidden within that answer... An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The short answer is the electrons, and the protons. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons have a positive electrical charge, so they are often represented with the mark of a … An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. 30.05.2021 · answered may 30, 2021. Hi, thanks for your question. It has a charge of +1.6 ×10−19c. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The center of the atom is called the nucleus... Hi, thanks for your question.

30.05.2021 · answered may 30, 2021. This is a positively charged particle that is present in the nucleus of atoms. The image above is the bohr model of the atom. This particle has a charge of zero; However there are a couple of subtilties hidden within that answer. 30.05.2021 · answered may 30, 2021. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Here we see a central nucleus containing the protons and. The center of the atom is called the nucleus. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons have a positive electrical charge, so they are often represented with the mark of a …. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.

Here we see a central nucleus containing the protons and. Here we see a central nucleus containing the protons and. Protons have a positive electrical charge, so they are often represented with the mark of a ….. Protons have a positive (+) charge.

It has a charge of +1.6 ×10−19c... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The image above is the bohr model of the atom. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Hi, thanks for your question. The image above is the bohr model of the atom.
The short answer is the electrons, and the protons. Protons have a positive electrical charge, so they are often represented with the mark of a … This particle has a charge of zero; It is present in the nucleus of atoms. Atoms are composed of particles called electrons, protons and neutrons. Protons have a positive (+) charge. This is a negatively charged … The three main subatomic particles that form an atom are protons, neutrons, and electrons. Hi, thanks for your question.

Atoms are composed of particles called electrons, protons and neutrons.. The three main subatomic particles that form an atom are protons, neutrons, and electrons. However there are a couple of subtilties hidden within that answer. What two particles determine the charge of an atom? It is present in the nucleus of atoms. Earlier it was postulated by john dalton that atoms are indivisible particles. Earlier it was postulated by john dalton that atoms are indivisible particles.

The three main subatomic particles that form an atom are protons, neutrons, and electrons.. Protons have a positive electrical charge, so they are often represented with the mark of a …. The image above is the bohr model of the atom.

However there are a couple of subtilties hidden within that answer. The short answer is the electrons, and the protons. Protons have a positive electrical charge, so they are often represented with the mark of a … This is a negatively charged …
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
However there are a couple of subtilties hidden within that answer.. However there are a couple of subtilties hidden within that answer... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons have a positive (+) charge. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The center of the atom is called the nucleus. The image above is the bohr model of the atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. However there are a couple of subtilties hidden within that answer. What two particles determine the charge of an atom? Protons have a positive electrical charge, so they are often represented with the mark of a …. Here we see a central nucleus containing the protons and.

What two particles determine the charge of an atom? Atoms are composed of particles called electrons, protons and neutrons.. It is present in the nucleus of atoms.